Reactions of Benzene
Have you ever wondered what happens when TNT explodes? Let's take a closer look. TNT, or trinitrotoluene, is a highly explosive substance. When it detonates, a wave of pressure triggers a reaction that produces a lot of gas and heat. This makes it very dangerous. TNT has a low melting point, which means it can be used as a liquid, and it doesn't dissolve in water. Because of these properties, it's used in many different situations, from mining to military tasks in wet environments.
The main component of TNT is benzene, which is a type of hydrocarbon. The hydrogen and carbon atoms in TNT come from the benzene ring, while the nitrogen and oxygen atoms come from three nitrate groups attached to the ring. TNT also has a methyl group attached to the benzene ring.
But how do we go from a flat benzene molecule to the complex structure of TNT? To do that, we need to understand the reactions of benzene in organic chemistry. The first type of reaction we'll look at is electrophilic substitution, which involves adding an electrophile to the benzene ring. We'll then explore specific reactions such as nitration, chlorination, and Friedel-Crafts acylation. We'll also look at other reactions that benzene can participate in, such as combustion and hydrogenation.
By the end of this article, you'll know what an electrophile is, how to draw the mechanisms for electrophilic substitution reactions involving benzene, and why benzene doesn't participate in addition reactions. You'll also be able to write an equation for the combustion of benzene. So, let's get started!
What is benzene?
Before we go any further, let’s first remind ourselves about benzene.
Benzene is an aromatic hydrocarbon with the molecular formula . Aromatic molecules are also known as arenes.
Each carbon atom within benzene is bonded to two other carbon atoms and one hydrogen atom. This means that each carbon atom has a spare valence electron. We find these electrons in an area formed by overlapping pi orbitals above and below the benzene ring. The electrons can move freely within this area - we say that they are delocalised.
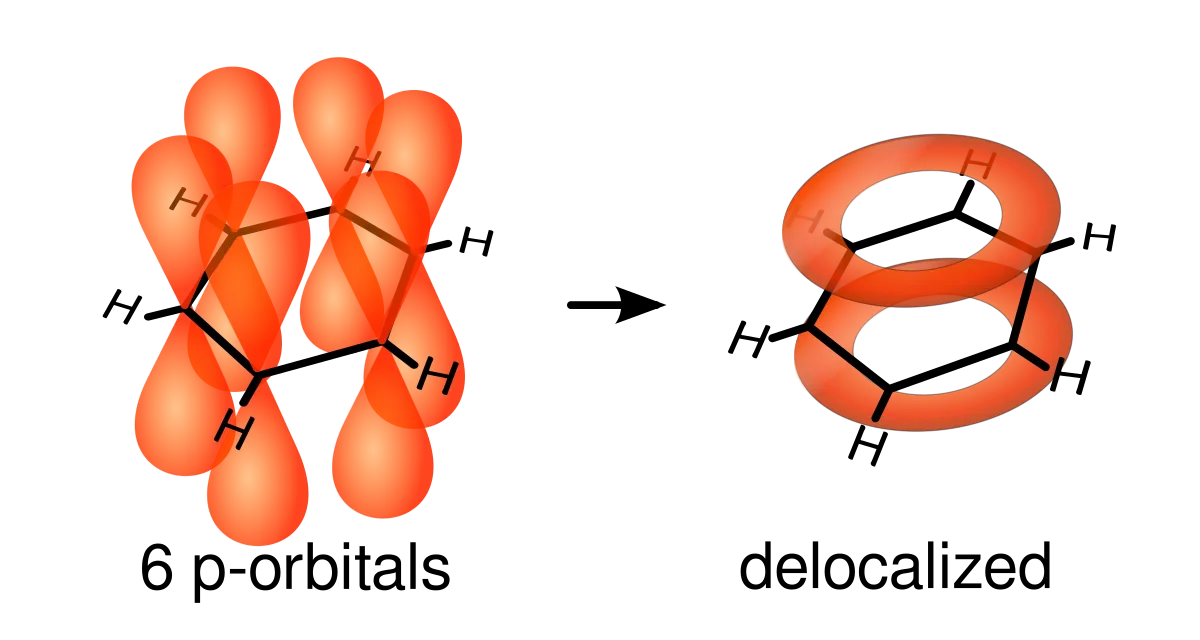
If you want a more in-depth explanation about the structure and bonding within benzene, check out Aromatic Chemistry and Benzene Structure.
How does benzene react?
As we explored above, benzene contains delocalised pi electrons found in a ring. This ring of delocalisation is relatively strong and stable because it spreads the charge of the electrons over a greater area. It takes a lot of energy to disrupt the delocalisation. Therefore, benzene doesn’t readily take part in reactions that involve breaking the ring, such as addition reactions. However, it does take part in substitution reactions. To be more precise, these tend to be electrophilic substitutions. Let’s break that term down a little.
What are substitution reactions?
Substitution reactions are reactions in which one atom, group of atoms or functional group is replaced by another on a molecule.
To 'substitute' just means to swap out one thing for another, which is exactly what happens in a substitution reaction. In benzene, substitution reactions involve getting rid of some of the hydrogen atoms attached to the carbon ring and replacing them with other, more useful groups of atoms. These could be nitrate groups or methyl groups.
What are electrophiles?
Electrophiles are electron pair acceptors. They have a vacant electron orbital and a positive or partially positive charge on one of their atoms.
Electrophiles are attracted to areas of high electron density - this just means areas with a lot of electrons crammed together. One example is benzene’s ring of delocalisation. It contains six electrons moving about randomly, free from any positive particles like protons, and so is a highly charged area. Electrophiles love it - after all, phile does come from the Latin term philos, meaning to love!
Because of this, benzene tends to take part in electrophilic substitution reactions.
Electrophilic substitution reactions of benzene
To start, let’s first look at the general mechanism of an electrophilic substitution reaction with benzene. The electrophile is represented by .

The electrophile is attracted to benzene’s ring of delocalisation. The electrophile forms a bond with one of benzene’s carbon atoms, using one of the ring’s electrons. This leaves benzene with a positive charge. The aromatic ring is now partially destroyed. Benzene would be a lot more stable if it repaired its electron ring. To do this, it breaks one of its C-H bonds. This releases the hydrogen atom as a ion. The spare electron returns to the ring of delocalisation, restoring it back to six electrons. The end product is a benzene derivative, where one of the hydrogen atoms has been replaced by the electrophile.
Notice the electron ring in the mechanism above. To show that it has been disrupted in the second step, we draw a broken circle. Remember to include the positive charge in the centre of the molecule.
There are three specific examples you should know that use this mechanism:
Nitration. Chlorination. We'll also look at bromination. Friedel-Crafts acylation. We'll look at this with acyl chlorides and acid anhydrides.
Each reaction has three steps:
The electrophile is generated.The electrophile reacts with benzene.The catalyst is regenerated.
We'll look at each of the reactions in turn.
Nitration of benzene
Do you remember TNT from the start of the article? It has three nitrate groups and one methyl group attached to a benzene ring. Nitrated arenes are also important industrially as they are the first step in making aromatic amines, used in products like dyes.

To nitrate arenes, we first need to produce the nitrate ion, NO2+. This acts as our electrophile. To do this, we mix concentrated sulfuric and nitric acids. Sulfuric acid is a stronger acid than nitric acid, so nitric acid is forced to act as a base - it accepts a proton given up by sulfuric acid. This forms nitrooxonium, NO2+H+. Nitrooxonium then breaks down into water and a nitronium ion, NO2+, as shown in the equation below:
NO2+H+ → H2O + NO2+
The nitronium ion is an electrophile. Remember, an electrophile is an electron pair acceptor with a vacant electron orbital and a positive or partially positive charge. The nitrate ion reacts with benzene because it is attracted to benzene’s ring of delocalisation, just like in the general mechanism shown above. The overall reaction involves heating benzene at 50 °C with concentrated sulfuric and nitric acids, using reflux to prevent any volatile components escaping. The end product is nitrobenzene.

During the nitration reaction, you'll notice that a hydrogen ion is released from benzene. This ion reacts with HSO4- to reform H2SO4. This means that the sulfuric acid is just a catalyst.
Here's an equation for the overall reaction:
C6H6 + HNO3 → C6H5NO2 + H2O
So how do we get from one nitrate group to three, as seen in TNT? Well, if you heat the reaction up to even higher temperatures, you increase the chance of further nitration reactions happening. Another hydrogen atom is replaced with a nitrate group. If we count the carbon atom with the original nitrate group as carbon 1, the second nitrate group tends to be directed towards carbon 3 or 5. This is because nitrate groups are electron withdrawing.

You might have also noticed the methyl group in TNT. A benzene ring with a methyl group attached is commonly known as toluene, and it reacts a lot faster than a benzene ring without any methyl groups. In fact, if you want to prevent further nitration reactions happening, you have to keep the temperature below 30 °C. Methyl groups are electron releasing and direct any nitrate groups towards positions 2, 4 and 6 in the benzene ring.
Chlorination of benzene
We can also swap hydrogen atoms on a benzene ring with chlorine atoms, using aluminium chloride as a catalyst. This is another type of electrophilic substitution reaction and uses the same general mechanism as nitration. Before the reaction can start, we first need to generate an electrophile to react with benzene. This is where our catalyst comes in. Aluminium chloride reacts with chlorine to form a positive chlorine cation and a negative aluminium tetrachloride ion: The chlorine cation can now react with benzene using the mechanism shown below. This forms chlorobenzene and a hydrogen ion:

In order for our catalyst to be effective, we need to regenerate it. Catalysts are substances that aren't used up in a reaction, and they maintain the same physical and oxidation state.
To regenerate the catalyst, we can use the aluminium tetrachloride that was produced when we generated the electrophile. The hydrogen ion, which was released when the chlorine cation reacted with benzene, reacts with aluminium tetrachloride to produce hydrochloric acid and reform our catalyst, aluminium chloride.
We can also use a similar process to brominate benzene. Instead of using chlorine gas, we can use bromine and the catalyst aluminium bromide.
Friedel-Crafts acylation using acyl chlorides
You might know from Acylation that acylation reactions involve adding the acyl group, , to another molecule. To acylate benzene, we heat an acid derivative, such as an acyl chloride or acid anhydride, with aluminium chloride at 60 °C. The reaction takes place in anhydrous conditions under reflux. Let's first focus on acylation using an acyl chloride. Like in nitration, we first need to generate an electrophile. In this case our electrophile is the positive cation; we form it by reacting our acyl chloride with aluminium chloride, our catalyst. The below equations show you the process, using R to represent an alkyl group. Notice the other product is once again a negative aluminium tetrachloride ion:
The electrophile reacts with benzene in much the same way as explored above. Again, it releases a hydrogen ion.

After the reaction, our product is a ketone with a benzene ring attached. We can use the prefix phenyl- to indicate this in the name.
For more information on naming organic compounds and aromatic chemistry, you can refer to resources like Organic Chemistry by Paula Yurkanis Bruice.
To regenerate aluminium chloride, the positive hydrogen ion released reacts with the negative aluminium tetrachloride ion that was formed earlier. This produces aluminium chloride and hydrochloric acid.
Here's the overall equation:
AlCl3 + HCl → AlCl4- + H+
Let's look at an example reaction of ethanoyl chloride with benzene. First, ethanoyl chloride reacts with aluminium chloride to form our negative anion, aluminium tetrachloride, and our electrophile, the positive acetyl cation.
The electrophile then reacts with benzene, producing phenylethanone and a hydrogen ion. You should be familiar with the mechanism for this reaction

Finally, the hydrogen ion reacts with the negative aluminium tetrachloride ion we formed earlier to regenerate the catalyst, also producing hydrochloric acid:
This gives us the following overall equation:
Friedel-Crafts acylation using acid anhydrides
Acid anhydrides can also react with benzene to produce a ketone with a benzene ring attached. The mechanism for this reaction is similar to the ones we've explored earlier, making it easier to remember.
First, we generate the electrophile using aluminium chloride and produce the positive acyl cation. This time, we produce a different negative ion.
The electrophile then reacts with benzene to produce a ketone and release a hydrogen ion. This is known as the Friedel-Crafts acylation reaction.
Finally, we regenerate the catalyst by reacting the positive hydrogen ion with the negative aluminium ion to produce aluminium chloride and hydrochloric acid.
Here's the overall equation:
By learning the general mechanism and applying it to different examples, you can get a better understanding of these reactions and how to use them in organic synthesis.
Summary
Phew - you made it through all the electrophilic substitution reactions! Here's a handy table to help summarise the new material:

In addition to electrophilic substitution reactions, benzene can also undergo several other types of reactions. Here are a few examples:
- Combustion: Benzene can burn in the presence of oxygen to produce carbon dioxide and water. The combustion of benzene is exothermic and can release a large amount of energy.
- Sulfonation: Benzene can react with sulfuric acid to form benzenesulfonic acid. This reaction can be used to introduce a sulfonic acid group onto the benzene ring, which can have various applications in organic synthesis.
- Hydrogenation: Benzene can be hydrogenated in the presence of a catalyst, such as platinum or palladium, to form cyclohexane. This reaction involves the addition of hydrogen to the benzene ring and can be used to convert benzene into a different type of organic compound.
While electrophilic substitution reactions are the most common type of reaction involving benzene, these other reactions also play important roles in organic synthesis and in the chemical industry.
Combustion
Benzene burns just like any other hydrocarbon to produce carbon dioxide and water. However, because of its high proportion of carbon, it often combusts incompletely. This produces a lot of carbon in the form of soot. Try writing an equation for the complete combustion of benzene. You should get the following:
Sulfonation
We can introduce the sulfonic acid group, , to benzene in another electrophilic substitution reaction. This is done, for example, by heating benzene with concentrated sulfuric acid under reflux. It forms white crystals of benzenesulfonic acid. Hydrogenation As the name suggests, hydrogenation involves adding hydrogen to a molecule. Hydrogenating benzene creates a cyclic alkane, cyclohexane. However, the reaction has a high activation energy as it involves breaking benzene’s stable ring of delocalised electrons. It uses a nickel catalyst and high temperatures and pressures. For example, hydrogenating methylbenzene would produce methylcyclohexane, as shown below:

To summarise, benzene is an aromatic compound with a strong ring of delocalised electrons. Most of the reactions of benzene are electrophilic substitution reactions, where an electrophile replaces a hydrogen atom on the benzene ring. Benzene can react with nitric acid to form nitrobenzene, with chlorine to form chlorobenzene, and with acid derivatives to form ketones. These reactions use various catalysts and conditions. Benzene can also undergo other reactions, such as sulfonation, combustion, and hydrogenation, which have important applications in organic synthesis and industry.
Reactions of Benzene
What type of reaction is bromination of benzene?
Bromination of benzene is a type of electrophilic substitution reaction.
What type of reactions does benzene undergo?
Benzene normally undergoes electrophilic substitution reactions. This is because addition reactions would involve disrupting its stable ring of delocalised electrons.
Why are addition reactions of benzene difficult?
Addition reactions of benzene are difficult because they would involve disrupting benzene's stable ring of delocalised electrons. This takes a lot of energy.
Why is the nitration of benzene a substitution reaction?
Nitration of benzene is a substitution reaction because a hydrogen atom from benzene is swapped for a nitrate group.
Does benzene give elimination reactions?
No, benzene doesn't normally give elimination reactions.








