Aromatic Chemistry
If you're interested in chemistry, you might have heard about the American Chemical Society. It's a scientific society based in Washington. Back in 1988, they compiled a list of chemicals and found that two-thirds of them contained a special type of ring - called a benzene ring. This is what makes them aromatic compounds.
Aromatic compounds are a type of organic molecule that have rings with electrons that move around, called delocalised pi electrons. Another term for them is "arenes".
In this article, we'll give you an introduction to aromatic compounds in organic chemistry. Firstly, we'll take a look at benzene and its structure. After that, we'll practice naming benzene derivatives. Finally, we'll explore how aromatic compounds are formed and touch on some of the reactions they take part in. If you're interested in learning more about Aromatic Chemistry, keep reading!
What is benzene?
Let's focus on molecules that have something in common - the benzene ring. Benzene is an aromatic compound that has six carbon atoms and six hydrogen atoms arranged in a flat ring shape.
The name "aromatic" comes from the fact that the first few of these compounds were found in sweet-smelling oils. Benzene, for example, was first isolated from benzoin, a resin made from certain Asian trees. However, not all sweet-smelling compounds are aromatic, and not all aromatic compounds smell good! Benzene is the most well-known aromatic compound, but there are others with rings of different sizes. One example is cyclotetradecaheptane, also called [14]annulene, which has 14 carbon atoms and 14 hydrogen atoms. There's even a rule for figuring out if a cyclic molecule is aromatic - it should have 4n+2 pi electrons, where n is a positive number. This rule is called Hückel's rule.
Shape
As we mentioned above, benzene is an aromatic hydrocarbon ring containing six carbon atoms and six hydrogen atoms. Try drawing it out and see what sort of structures you can come up with.

In actual fact, benzene has a completely different structure to all three molecules shown above. It doesn’t even contain a single double bond! Instead, each of benzene’s carbon atoms is bonded to just one hydrogen atom and two other carbon atoms, forming a hexagon. We give benzene the following symbol:

Bond length
If benzene doesn’t contain any double bonds, what sort of bonds does it have?
Each of benzene's carbon-carbon bonds is the same length, and is neither a single bond nor a double bond, but something in between. We call them intermediates. You can see this in the table below, which shows the lengths of different carbon bonds:
Delocalisation
When we count the electrons involved in benzene, we run into a problem. Carbon has four electrons in its outermost shell. In benzene, two of these electrons form bonds with adjacent carbon atoms to create sigma bonds. Another electron bonds with a hydrogen atom. This would leave one electron unaccounted for on each carbon atom.
However, this is where delocalisation comes in. The remaining electron on each carbon atom is found in a pi orbital. If you remember alkenes, sigma orbitals and bonds stretch between adjacent atoms, while pi orbitals go above and below each atom. In benzene, the pi orbitals of all six carbon atoms overlap and create one big region of electron density. This is where the electrons become delocalised. They can move freely within the region and don't belong to one particular carbon atom.

The three bonding electrons are actually found in special orbitals called orbitals. This is a little complicated and goes beyond what you’ll be tested on in an exam, but is interesting to know. Carbon has the electronic structure of . In terms of orbitals, its valence shell has a pair of electrons in the orbital and one electron each in two of the orbitals. However, to form three bonds, carbon needs three unpaired electrons. To do this, it enters an ‘excited’ state - it promotes one of the electrons from into the empty orbital.

We know that in benzene, carbon wants to form three bonds. These bonds are all equal. To make three equal bonds, carbon needs three electrons in equal orbitals. The easiest way for it to do this is to hybridise three of its orbitals: and . These form three identical orbitals known as orbitals, because - you guessed it - they are made from one s orbital and 2 p orbitals.
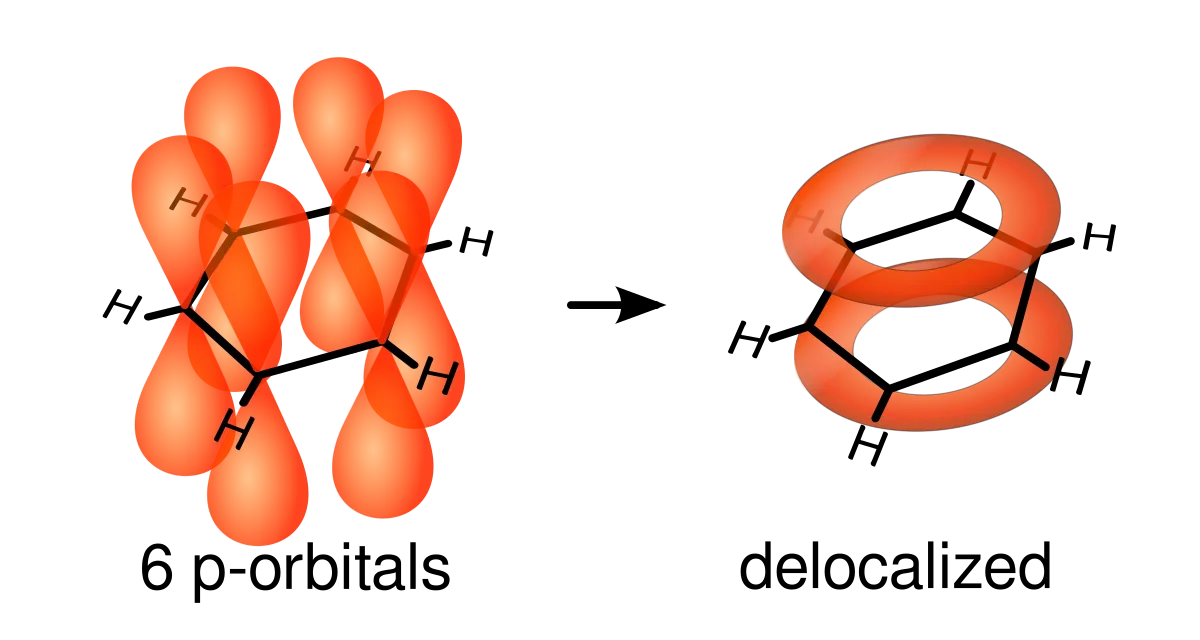
If you aren’t sure about orbitals, see Electron Shells, Subshells and Orbitals.
Bond angle
Each of benzene’s carbon atoms has three bonds: two C-C bonds and one C-H bond. These bonds try to spread themselves out as far apart as possible. This results in an angle of 120° between each bond. Therefore, benzene forms a trigonal planar molecule.
Properties of benzene
Benzene has some interesting properties that we'll explore more in-depth under the topic of Structure and Bonding, but here are a few key things to know:
- Benzene is a planar molecule with bond angles of 120°, making it trigonal planar in shape.
- Its flat structure allows for close packing of molecules, resulting in relatively high melting and boiling points.
- Benzene's electron ring gives it stability compared to other hydrocarbons, which is known as aromatic stability.
- Despite being unsaturated, benzene resists addition reactions.
Understanding the properties of benzene is important in organic chemistry, as it is a building block for many more complex compounds.
How do you name aromatic compounds?
Now that we know what benzene is, we can now look at naming different molecules containing its characteristic ring.
Benzene derivatives use the suffix -benzene. However, if there are multiple functional groups present they sometimes use the prefix phenyl- instead. Let’s look at some examples to remind ourselves of nomenclature rules. If you need a quick reminder before we begin, look at Organic Compounds.

In this molecule, there is a methyl group and a chlorine atom attached to the benzene ring. To name it, we use the prefixes methyl- and chloro-. We also need to use numbers to indicate the positions of the functional groups on the carbon ring. However, since benzene has no end of the chain, we can start numbering from any of the carbons and follow the 'lowest number' rule. In this case, we can label the carbon attached to the methyl group as number 1, and the carbon attached to the chlorine atom as number 3. Following alphabetical order, we would name the molecule as 3-chloro-1-methylbenzene.

Here’s another example.

It is actually just a ketone, where one of the R groups is a benzene ring. We have to use the prefix phenyl-. The remaining carbon chain is 2 atoms long, taking the root name -eth-, so this molecule is known as phenylethanone.
Try this one?

This next molecule has a carboxyl group and a hydroxyl group attached to its benzene ring. We’ll need to use the suffix -oic acid and the prefix hydroxy-. Counting the carbon atom attached to the carboxyl group as carbon 1, the carbon atom containing the hydroxyl group takes position 2. We therefore call this molecule 2-hydroxybenzoic acid.

A benzene ring with just a hydroxyl group attached has its own special name: phenol.

How do you form aromatic compounds?
To make benzene rings and other aromatic compounds, we use a process called catalytic reforming. To do this, we take fractions from crude oil that are around six to eight carbon atoms long. We then heat them with a catalyst and hydrogen gas to 500 °C at a pressure of about 20 atm. The catalyst is a mixture of aluminium oxide and platinum. This is why the process is sometimes known as ‘platforming’. At such high temperatures, some of the hydrocarbons tend to decay into carbon, which contaminates the catalyst, but adding hydrogen stops this process. The products are benzene derivatives and more hydrogen gas.

How do aromatic compounds react?
Take a look at benzene again. It is an unsaturated molecule. We’ve met that term before when describing alkenes with C=C double bonds. Although benzene doesn’t have any double bonds, it doesn’t contain the full possible number of hydrogen atoms. Each carbon atom can potentially bond to two other carbon atoms and two hydrogen atoms, which would make the saturated cyclic hydrocarbon called cyclohexane, .

Benzene is unique among unsaturated compounds, like alkenes, in that it does not readily undergo addition reactions. This is because addition reactions would break the ring of delocalized electrons, which requires a lot of energy. Instead, benzene typically undergoes substitution reactions, where one atom or group is replaced by another.
The ring of delocalized electrons in benzene has a high electron density, which makes it attractive to electrophiles. Electrophiles are electron pair acceptors that have an empty orbital and a positive or partial positive charge. For this reason, benzene often undergoes electrophilic substitution reactions. Some examples of electrophilic substitution reactions include nitration reactions, where a hydrogen atom is replaced with a nitro group to produce nitrobenzene, which is used in dyes and pharmaceuticals. Another example is Friedel-Crafts acylation reactions, where benzene reacts with an acid derivative in the presence of an aluminum chloride catalyst to produce a product used in plastics and detergents. Benzene has a high carbon-to-hydrogen ratio, which means that when it burns, it produces a sooty flame. This is one way to identify aromatic compounds.
To summarize, here are the key takeaways from the topic of aromatic chemistry:
- Aromatic compounds, also called arenes, contain a ring with delocalized pi electrons. The most common aromatic ring is benzene.
- Benzene is a hexagonal ring of six carbon atoms with identical bonds between each carbon atom.
- Each carbon atom in benzene contains one unbonded electron that forms a pi orbital. These orbitals overlap above and below the ring to form a region of delocalization called a ring of aromaticity.
- Benzene is produced from crude oil fractions using an aluminum oxide and platinum catalyst at high temperature and pressure.
- Benzene is relatively stable and has higher melting and boiling points compared to alkanes.
- We name benzene derivatives using the suffix -benzene or the prefix phenyl-.
- Benzene often undergoes electrophilic substitution reactions such as nitration and Friedel-Crafts acylation reactions.
Aromatic Chemistry
What is aromaticity in chemistry?
Aromatic compounds are compounds that contain a ring with delocalised pi electrons. The most common aromatic compound is benzene, a ring made from six carbon atoms and six hydrogen atoms.
What is an aromatic ring?
An aromatic ring is a ring of carbon atoms with delocalised pi electrons. Each carbon atom forms three bonds: two C-H bonds and one C-H bond. Carbon’s fourth electron is found in a pi orbital. This electron delocalises, and all of the delocalised electrons move into a region above and below the ring. This makes benzene more stable.
What compounds are aromatic?
All benzene derivatives are aromatic compounds. Examples include chlorobenzene and nitrobenzene. Other examples of aromatic compounds are vanillin and cinnamaldehyde, the main constituents of vanilla and cinnamon respectively.
How do you know if a compound is aromatic?
Aromatic compounds all contain rings with delocalised pi electrons, such as the benzene ring.








