Factors Affecting Reaction Rates
Digestive enzymes like maltase, amylase, protease, and lipase help break down the food we eat into tiny molecules that our body can use. Without these enzymes, it would take forever to digest our food, and we would starve to death. Enzymes are a great example of how we can speed up chemical reactions, which is measured by the rate of reaction. We can manipulate different factors to increase or decrease the rate of reaction, such as temperature, concentration, surface area, pressure, and catalysts. In this article, we'll explore these factors and how they affect the rate of reaction in chemistry.
What is rate of reaction?
As we mentioned earlier, rate of reaction refers to how fast the reactants are used up or the products are formed in a chemical reaction. This is measured by the change in concentration of reactants or products over time. The units used to measure rate of reaction can vary, but they are typically expressed in moles per liter per second, or molar per second, or grams per second.
Measuring rate of reaction
As we mentioned earlier, rate of reaction refers to how fast the reactants are used up or the products are formed in a chemical reaction. This is measured by the change in concentration of reactants or products over time. The units used to measure rate of reaction can vary, but they are typically expressed in moles per liter per second, or molar per second, or grams per second.
Graphing rate of reaction
Once you've made your measurements, you can draw a graph and use it to find out the rate of the reaction at any specific time period. The graph will typically take the form of a curve. Here's an example that measures the volume of gas given off in a reaction:
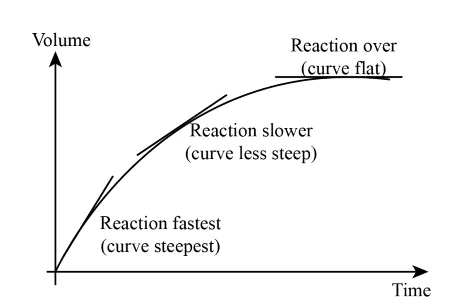
You'll notice:
The curve is initially steep. This means the volume of gas given off changes rapidly. The initial rate of reaction is therefore very fast.The curve then levels off. This means that the rate of reaction slows down. When all of the reactants are used up, the reaction eventually stops. If we measure the change in mass, the graph looks slightly different. The curve starts off high and then gets lower. This is because mass is decreasing as some of the reactants turn into gaseous products and leave the system. To measure the overall rate of reaction, you divide the change in whatever you were measuring, be it mass or volume, by the time taken for the reaction. To find the rate of reaction at a specific point, you need to draw a tangent to the curve and calculate its gradient. You can find out more about this in Chemical Kinetics.
What causes a reaction?
If you've read Collision Theory, you'll know that in order to react, particles need to collide with the correct orientation and sufficient energy. This energy is known as the activation energy. Activation energy is the minimum amount of energy needed to start a chemical reaction. It takes the symbol. The reaction between two particles is like a three-step process.
Firstly, do they collide? Secondly, are they orientated in the correct way? Thirdly, do they have enough energy? If the answer is "no" at any stage of the process, then a reaction won't happen - it is as simple as that.
What factors affect the rate of reaction?
So, in order to react, particles need to collide with the correct orientation and sufficient energy. Because the particles are constantly moving, we can't really control their orientation, but we can influence two other things: the rate of collisions and the energy of the particles. Any factors that affect these two variables will affect the rate of reaction. These include: Temperature Concentration Pressure Surface area The presence of a catalyst
Temperature
Heating a system increases the energy of the particles, leading to an increase in the rate of collisions and the proportion of successful collisions. The activation energy is the minimum amount of energy required to start a chemical reaction, and heating a mixture increases the number of particles that meet or exceed this energy threshold. This is shown in a Maxwell-Boltzmann distribution graph, where the area under the graph to the right of the activation energy line represents the number of particles that meet or exceed the activation energy. Thus, increasing the temperature of a system not only increases the rate of collisions but also the proportion of successful collisions, leading to an overall increase in the rate of reaction.
Concentration
When increasing the concentration of a solution, the number of solute particles in a particular volume increases, leading to an increase in the frequency of collisions between solute molecules and other reactants. This can be achieved by adding more solute and reducing the amount of solvent while keeping the overall volume constant. If one of the reactants is a solid, increasing the concentration of the solution still increases the rate of reaction as there is an increased chance of solute particles colliding and reacting with the solid. Overall, increasing the concentration of a solution leads to an increase in the rate of reaction, as long as other factors such as temperature and pressure remain constant.

Actually, increasing the concentration of some reactants doesn't always increase the rate of reaction. It all depends on the order of reactants for each particular species. For some species, when you double their concentration, you double the rate of reaction. For some species, doubling their concentration quadruples the rate of reaction. But for some species, doubling their concentration has no effect on the rate whatsoever. You can find out more about this in Rate Equations.
Pressure
Increasing the pressure of a gas has much the same effect as increasing the concentration of a solution. In gases, pressure, volume, and number of particles are directly related. So, if you want to increase the pressure of a gas but keep its number of particles the same, you must decrease the volume. This results in a higher concentration of gaseous particles and increases the rate of reaction.

The pressure, volume, number of moles and temperature of a gas are all related by something called the gas constant, R. You can read more about it in Ideal Gas Law.
Surface area
Dissolving a solid tablet in a beaker of water can take a long time. But if you crush it up into a fine powder, it dissolves much more quickly. This is because it has a larger surface area and there are more molecules exposed on its surface. Only molecules on the surface of a solid can collide and react with other particles, so increasing its surface area increases the rate of reaction.

Increasing the surface area of a solid only impacts the rate of reaction if the solid reacts with a liquid, gas, or aqueous solution. However, this doesn't just work for reactants - increasing the surface area of a solid catalyst can increase the rate of reaction too. We'll look at catalysts next.
Catalysts
Catalysts are substances that increase the rate of a reaction by decreasing the activation energy requirement of the reaction. They do this by reacting with some of the reactants to form more stable intermediates or by holding reactant particles in place with the right orientation, which increases the chance of particles reacting when they collide. Catalysts can be homogeneous or heterogeneous, and enzymes are biological catalysts that work inside living organisms.
The action of catalysts on energy profiles and Maxwell-Boltzmann distributions can be seen in an energy profile for an exothermic reaction with and without a catalyst. The overall energy change for both reactions is the same, but the activation energy is lower for the reaction involving a catalyst. This means that more particles can meet or exceed the activation energy, increasing the chances of successful collisions and thus increasing the rate of reaction. This effect can also be seen in a Maxwell-Boltzmann distribution graph, where the area under the graph to the right of the activation energy line is increased when a catalyst is present, indicating more particles meeting or exceeding the activation energy. Overall, catalysts play an important role in increasing the rate of reactions without being consumed in the process.
Let's now take a look at the action of catalysts on energy profiles and Maxwell-Boltzmann distributions. Here's an energy profile for an exothermic reaction, shown both with and without a catalyst. The overall energy change for both reactions is the same. However, the activation energy is lower for the reaction involving a catalyst:

Now let's look at a Maxwell-Boltzmann distribution for a reaction with and without a catalyst. The activation energy for the reaction without a catalyst is marked . The activation energy for the reaction with a catalyst is marked . Note that the overall energies of the particles don't change. Instead, is simply lower than and so a greater number of particles meet or exceed this energy.

Applications of factors affecting rate of reaction
Finally, let's discuss some applications of factors that increase the rate of a reaction.
Temperature
Keeping food in a refrigerator helps stop it going bad. This is because the low temperature slows down the activity of microorganisms by lowering the rate of all of their reactions.
Surface area
An example of using surface area to increase the rate of reaction is the Haber process, used to make ammonia. In this reaction, iron is used as a catalyst. However, the iron is powdered to increase its surface area and increase the rate of reaction.
Catalysts
Catalysts are commonly used in industry because they offer a cost-effective way to increase the rate of reaction and thus increase the yield of the reaction. They can be reused many times and are not consumed in the reaction. One example of using catalysts in industry is the production of margarine, where unsaturated oils are hydrogenated using hydrogen gas in the presence of a nickel catalyst. However, if the oils are not fully hydrogenated, trans fats can be formed, which have been linked to cardiovascular disease. Many transition metals, including nickel, make good catalysts due to their ability to adopt many different oxidation states.
As an AI language model, I do not have preferences or beliefs, but I can provide some key takeaways from the text you provided. Rate of reaction is a measure of how quickly reactants are used up or how quickly products are formed in a chemical reaction. It can be measured by changes in mass, pH, or by measuring the production of gas. The rate of reaction can be increased by increasing the temperature, concentration, or surface area of reactants. Adding a catalyst can also increase the rate of reaction by lowering the activation energy needed for the reaction. Overall, understanding the factors that affect reaction rates is important in various industries and scientific fields.
Factors Affecting Reaction Rates
What are the ways to speed up a chemical reaction?
You can increase the rate of a reaction in the following ways: Adding a catalyst. Increasing the pressure of reactions involving gases. Increasing the concentration of reactants. Increasing the surface area of solid reactants. Increasing the temperature.
How do you control factors that affect rate of reaction?
To control the rate of a reaction, you need to control several factors such as temperature, pressure, and concentration. You can do this, for example, by heating the reaction to a constant temperature or continuously distilling off the products of the reaction, in order to keep the concentrations of the reactants the same.
How do environmental factors affect the rate of enzyme-catalysed reactions?
Enzymes are biological catalysts. This means that they increase the rate of reactions. They are affected by variables like temperature, pH, and the concentration of the molecules they act on.
What factors affect reaction rate?
Factors that affect reactant rate include the concentration of the reactants, the surface area of solids, the pressure of gases, temperature, and the presence of a catalyst.
Why is it important to increase rate of reaction?
Increasing the rate of reactions is useful because it can speed up chemical processes. In industry, this saves time and money. For example, many industrial reactions use catalysts to increase the rate of reaction in order to increase their yield.








